1.1

Description *(diagnosis (if known) or signs/symptoms)*
1.2

Start date
1.3

Ongoing at end of study
1.4

End date
Only shown if field 1.3 is equal to 2

**NOTE: End date adverse event is before start date adverse event. Please adjust!**
Only shown if field 1.30 is equal to 1
1.6

Intensity
1.7

Relation with study medication
1.8

Action study medication
1.9

Specify other study medication action
Only shown if field 1.8 is equal to 6
1.10

Other action *(check all that apply)*
1.11

Specify other other action
Only shown if field 1.10 is equal to 4
1.12

Is the Adverse Event reduced after discontinuation of the study medication or adjustment of the dose?
1.13

Is the study medication re-administered or is the adjusted dose returned to the original dosage?
Only shown if field 1.12 is equal to 1
1.14

Date of administration/dose return to original dosage
Only shown if field 1.13 is equal to 1
1.15

Did the Adverse Event recur after restarting the study medication or after the adjusted dose was returned to the original dosage?
Only shown if field 1.13 is equal to 1
1.16

Date Adverse Event recurred
Only shown if field 1.15 is equal to 1
1.17

Outcome
1.18

If stabilized, stabilization date
Only shown if field 1.17 is equal to 2
1.19

Specify residual symptoms
Only shown if field 1.17 is equal to 4
1.20

Date of death
Only shown if field 1.17 is equal to 5
1.21

Serious Adverse Event?
1.22

(check 1 or more answer options)
Only shown if field 1.21 is equal to 1
1.23

Admission date
Only shown if field 1.22 is equal to 3
1.24

Discharge date
Only shown if field 1.22 is equal to 3

**NOTE: Hospital discharge date is before hospital admission date. Please adjust!**
Only shown if field 1.31 is equal to 1
1.26

Specify other event
Only shown if field 1.22 is equal to 6

**Note: "Hospitalisation" is not checked for Other action AND for Specification SAE**
Only shown if field 1.32 is equal to 0
1.28

Expected?

**Potential SUSAR.** See "https://english.ccmo.nl/investigators/clinical-trials-with-medicinal-products-ctr/conduct-of-study-ctr/safety-reporting/serious-adverse-events-and-susars" for more information on reporting a SUSAR
Only shown if field 1.33 is equal to 1
1.30

Is stop datum vóór start datum adverse event?
1.31

Is ontslag datum vóór opname datum?
1.32

Cross check 'ziekenhuisopname' Overig vs SAE
1.33

SUSAR check
2.1

Description *(diagnosis (if known) or signs/symptoms)*
2.2

Start date
2.3

Ongoing at end of study
2.4

End date
Only shown if field 2.3 is equal to 2

**NOTE: End date adverse event is before start date adverse event. Please adjust!**
Only shown if field 2.28 is equal to 1
2.6

Intensity
2.7

Relation with study intervention
2.8

Action study treatment
2.9

Specify modified action
Only shown if field 2.8 is equal to 4
2.10

Other action *(check all that apply)*
2.11

Specify other other action
Only shown if field 2.10 is equal to 4
2.12

Is the Adverse Event reduced after discontinuation or modification of study treatment?
2.13

Is the study treatment resumed or is the adjusted study treatment returned to the original study treatment?
Only shown if field 2.12 is equal to 1
2.14

Date of resumption/reduction of study treatment
Only shown if field 2.13 is equal to 1
2.15

Did the Adverse Event recur after restarting study treatment or after the modified study treatment was returned to the original study treatment?
Only shown if field 2.13 is equal to 1
2.16

Date Adverse Event reccurred
Only shown if field 2.15 is equal to 1
2.17

Outcome
2.18

If stabilized, date of stabilization
Only shown if field 2.17 is equal to 2
2.19

Specify residual symptoms
Only shown if field 2.17 is equal to 4
2.20

Date of death
Only shown if field 2.17 is equal to 5
2.21

Serious Adverse Event?
2.22

(check 1 or more answer options)
Only shown if field 2.21 is equal to 1
2.23

Admission date
Only shown if field 2.22 is equal to 3
2.24

Discharge date
Only shown if field 2.22 is equal to 3

NOTE: Discharge date of hospitalisation is before admission date of hospitalisation. Please adjust!
Only shown if field 2.29 is equal to 1
2.26

Specify other event
Only shown if field 2.22 is equal to 6

NOTE: "Hospitalisation"; is not checked for Other action AND for Specification SAE
Only shown if field 2.30 is equal to 0
2.28

Is stop datum vóór start datum?
2.29

Is opname datum vóór ontslag datum?
2.30

Cross check 'ziekenhuisopname' Overig vs SAE
3.1

Description *(diagnosis (if known) or signs/symptoms)*
3.2

Start date
3.3

Ongoing at end of study
3.4

End date
Only shown if field 3.3 is equal to 2

**NOTE: End date adverse event is before start date adverse event. Please adjust!**
Only shown if field 3.30 is equal to 1
3.6

Intensity
3.7

Relation with study medication
3.8

Action medical device
3.9

Specify other action medical device
Only shown if field 3.8 is equal to 6
3.10

Other action *(check all that apply)*
3.11

Specify other other action
Only shown if field 3.10 is equal to 4
3.12

Is the Adverse Event reduced after discontinuation of the study medication or adjustment of the dose?
3.13

Is the study medication re-administered or is the adjusted dose returned to the original dosage?
Only shown if field 3.12 is equal to 1
3.14

Date of administration/dose returned to original dosage
Only shown if field 3.13 is equal to 1
3.15

Did the Adverse Event recur after restarting the medical device or after the adjusted dose was returned to the original dosage?
Only shown if field 3.13 is equal to 1
3.16

Date Adverse Event recurred
Only shown if field 3.15 is equal to 1
3.17

Outcome
3.18

If stabilized, stabilization date
Only shown if field 3.17 is equal to 2
3.19

Specify residual symptoms
Only shown if field 3.17 is equal to 4
3.20

Date of death
Only shown if field 3.17 is equal to 5
3.21

Serious Adverse Event?
3.22

(check 1 or more answer options)
Only shown if field 3.21 is equal to 1
3.23

Admission date
Only shown if field 3.22 is equal to 3
3.24

Discharge date
Only shown if field 3.22 is equal to 3

NOTE: Discharge date of hospitalisation is before admission date of hospitalisation. Please adjust!
Only shown if field 3.31 is equal to 1
3.26

Specify other event
Only shown if field 3.22 is equal to 6

**NOTE: "Hospitalisation" is not checked for Other action AND for Specification SAE**
Only shown if field 3.32 is equal to 0
3.28

Expected?

**Potential USADE.** See "https://www.ccmo.nl/onderzoekers/publicaties/publicaties/2021/05/08/flowchart-ongewenste-voorvallen-onderzoek-met-medische-hulpmiddelen" for more information on reporting a USADE
Only shown if field 3.33 is equal to 1
3.30

Is stop datum vóór start datum adverse event?
3.31

Is ontslag datum vóór opname datum?
3.32

Cross check 'ziekenhuisopname' Overig vs SAE
3.33

USADE check
1.1

Medication name *(brand/generic name)*
1.2

Indication
1.3

Dose per administration
1.4

Unit
1.5

Specify other unit:
Only shown if field 1.4 is equal to 33
1.6

Frequency
1.7

Specify other frequency:
Only shown if field 1.6 is equal to 16
1.8

Route
1.9

Specify other route:
Only shown if field 1.8 is equal to 26
1.10

Was the participant already receiving this concomitant medication prior to the study?
1.11

Start date
Only shown if field 1.10 is equal to 2
1.12

Ongoing at the end of study
1.13

End date
Only shown if field 1.12 is equal to 2
1.14

Is eind datum vóór start datum medicatie?

**NOTE: End date of medication is before the start date of medication. Please adjust!**
Only shown if field 1.14 is equal to 1
1.1

Medication name
1.2

Frequency
1.3

Specify other frequency:
Only shown if field 1.2 is equal to 16
1.4

Dosage *(for example 80 mg)*
1.5

Unit
1.6

Specify other unit:
Only shown if field 1.5 is equal to 33
1.1

Deviation number
1.2

Date deviation discovered
1.3

Date deviation occured
1.4

At what level did the deviation occur? *(check all that apply)*
1.5

Type of deviation
1.6

Specify other type of deviation
Only shown if field 1.5 is equal to 10
1.7

Participant number _(if applicable)_
1.8

Description of the deviation
1.9

Deviation (potentially) impact on participant
1.10

Deviation (potentially) impact on study data
1.11

Classification
1.12

Please complete the deviation form.
Only shown if field 1.11 is not 1
1.1

Investigational (medicinal) product (I(M)P) name:
1.2

*Dose prescribed - frequency per day*
1.3

*Dose prescribed - amount*
1.4

*Dose prescribed - dosage per time*
1.5

*Route of administration*
1.6

Specify other route
Only shown if field 1.5 is equal to 26
1.7

Batchnumber
1.8

Expiry date

**DAY 1**
1.10

Date

*Morning*
1.12
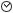
Time of administration / I(M)P taken *(24h clock)*
1.13

Number taken/administered
1.14

Administered / taken dose
1.15

Compliant?

*Midday*
1.17
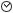
Time of administration / I(M)P taken - midday *(24h clock)*
1.18

Number taken/administered
1.19

Administered / taken dose
1.20

Compliant?

*Evening*
1.22
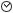
Time of administration / I(M)P taken - evening *(24h clock)*
1.23

Number taken/administered
1.24

Administered / taken dose
1.25

Compliant?

**DAY 2**
1.27

Date

*Morning*
1.29
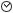
Time of administration / I(M)P taken *(24h clock)*
1.30

Number taken/administered
1.31

Administered / taken dose
1.32

Compliant?

*Midday*
1.34
Time of administration / I(M)P taken - midday *(24h clock)*
1.35

Number taken/administered
1.36

Administered / taken dose
1.37

Compliant?

*Evening*
1.39
Time of administration / I(M)P taken - evening *(24h clock)*
1.40

Number taken/administered
1.41

Administered / taken dose
1.42

Compliant?

**TOTAL INTAKE and % COMPLIANCE**
1.44

Total intake
1.45

% compliance
1.1

Date
1.2

Treatment received
1.1

Deviation has impact on:
1.2

Date deviation occurred
1.3

At what level did the deviation occur? *(check all that apply)*
1.4

Date of awareness

**Reported by:**
1.6

- Name
1.7

- Role
1.8

Type of deviation
1.9

Specify other type of deviation
Only shown if field 1.8 is equal to 10
1.10

Description of the deviation
1.11

Reason for the deviation (root cause analysis)
1.12

Corrective action(s) taken to resolve this occurrence?
1.13

Preventive action(s) taken to prevent future occurrence?
1.14

Calculation action specify
1.15

If yes, what type of action(s)? *(check all that apply)* ***You may add more details of action(s) taken in section ‘Comments’ below.***
Only shown if field 1.14 is equal to 1
1.16

Specify last visit to be performed:
Only shown if field 1.15 is equal to 2
1.17

Specify other action
Only shown if field 1.15 is equal to 11
1.18

In case of a medicinal study under CTR, is this deviation a serious breach?(3)
1.19

Corrective & preventative action (CAPA)
1.20

Comments
2.1

Deviation has impact on:
2.2

Name local principal investigator (PI)
2.3

Date deviation occurred
2.4

At what level did the deviation occur? *(check all that apply)*
2.5

Date awareness

**Reported by:**
2.7

- Name
2.8

- Role
2.9

Type of deviation
2.10

Specify other type of deviation
Only shown if field 2.9 is equal to 10
2.11

Description of the deviation
2.12

Reason for the deviation (root cause analysis)
2.13

Corrective action(s) taken to resolve this occurrence?
2.14

Preventive action(s) taken to prevent future occurrence?
2.15

Calculation action specify
2.16

Corrective & preventative action (CAPA)
Only shown if field 2.15 is equal to 1

The following questions should be completed by the coordinating principal investigator*. _(*the coordinating principal investigator has the final responsibility for the study as a whole, it is important to have his/her assessment of the impact of the deviation.)_
3.2

Does this deviation affect the participant’s safety, rights or well-being?
3.3

Explain your choice:
3.4

Does this deviation affect the reliability and robustness of the study data?
3.5

Explain your choice:
3.6

Corrective action(s) taken to resolve this occurrence?
3.7

Preventive action(s) taken to prevent future occurrence?
3.8

Calculation action specify
3.9

If yes, what type of action(s)? *(check all that apply)* ***You may add more details of action(s) taken in section ‘Comments’ below.***
3.10

Specify last visit to be performed:
Only shown if field 3.9 is equal to 2
3.11

Specify other action:
Only shown if field 3.9 is equal to 11
3.12

In case of a medicinal study under CTR, is this deviation a serious breach?(3)
3.13

Comments
1.1

Vaccination *(name of vaccine against)*
1.2

Vaccination date
Med_unit
Med_freq
Med_route
YesNo
Intensity AE
Relation AE
Action AE
Other action AE
YesNoNa
YesNoUnk
Outome AE
Spec SAE
Action - ND
Deviation level(AUMC)
Deviation_type
Classification deviation
Deviation_impact
Action type deviation
Serious deviation
Deviation level(local)